Audit Checklist for the Pharmaceutical Industry: 6 Steps to Stay on Track
Our pharmaceutical audit checklist has everything you need to know for the next inspection at your facility or office!
Language: English
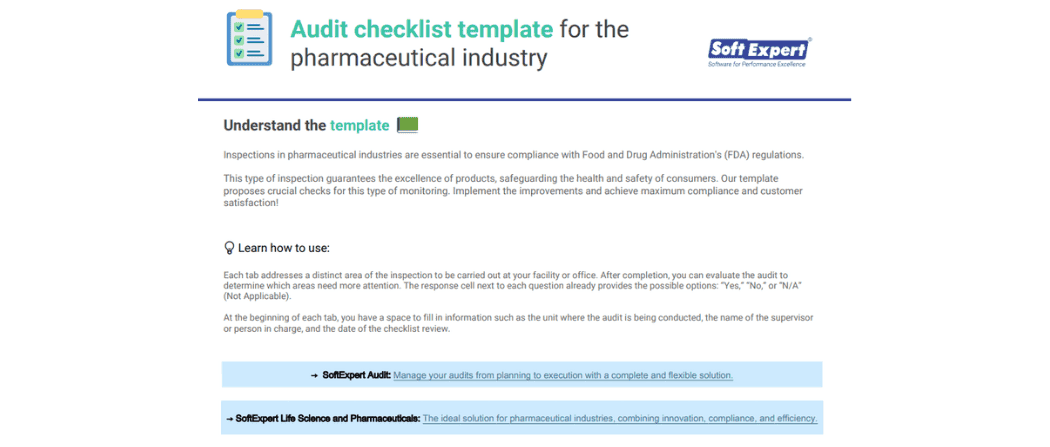
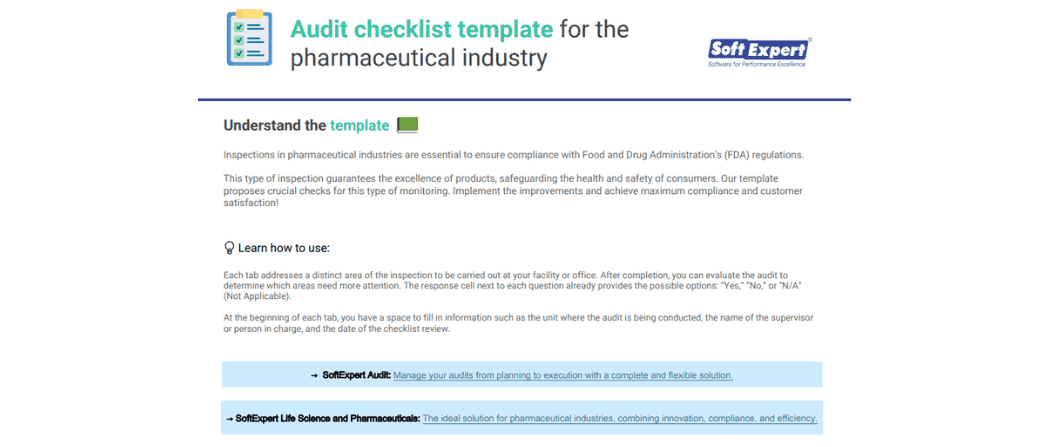
About the content
A complete audit checklist for the pharmaceutical industry, providing six essential steps to ensure you stay on track. Created based on Food and Drug Administration’s (FDA) guidelines, it covers the most important areas to pay attention to during an inspection at your facility or office.
Our spreadsheet will help you:
• Identify non-conformities and adverse events
• Perform root cause analysis
• Manage Corrective Actions (CAPA)
• Manage preventive actionsMonitor the internal quality audit/BPF program
• Conduct audits and evaluate effectiveness
Download our audit checklist and start preparing now for the next inspection!
Share this resource